HUABIO continuously pursues the specificity, stability, and reproducibility of antibody products. Based on the core HiMab recombinant rabbit monoclonal antibody R & D technology and using the “5+1” antibody validation strategy as the verification standard, we provide high - quality and batch - stable antibody products and efficient services for scientific researchers.
HUABIO Product Quality Standard System
“5+1” Antibody Validation Strategy
We follow the “Antibody Validation Protocol” (Nature Methods) published by the International Working Group for Antibody Validation (IWGAV) in 2016. Meanwhile, based on our decades of experience in antibody R & D and production, we have developed a unique antibody production validation strategy - the “5+1” strategy. This strategy is used to determine the functionality, specificity, and sensitivity of antibodies, strictly control antibody quality, and continuously provide customers with better antibody products.
Gene Strategy
Use technologies such as CRISPR/Cas9 or RNAi to knock out or knock down the target gene. Validate antibody specificity by comparing the absence or reduction of relevant protein signals in control cells or tissues.

Biological Characteristics and Orthogonal Strategy
Conduct qualitative and quantitative validation based on biological characteristics, or use non - antibody - dependent methods to detect the correlation between antibody - based and biological characteristics or non - antibody quantification.
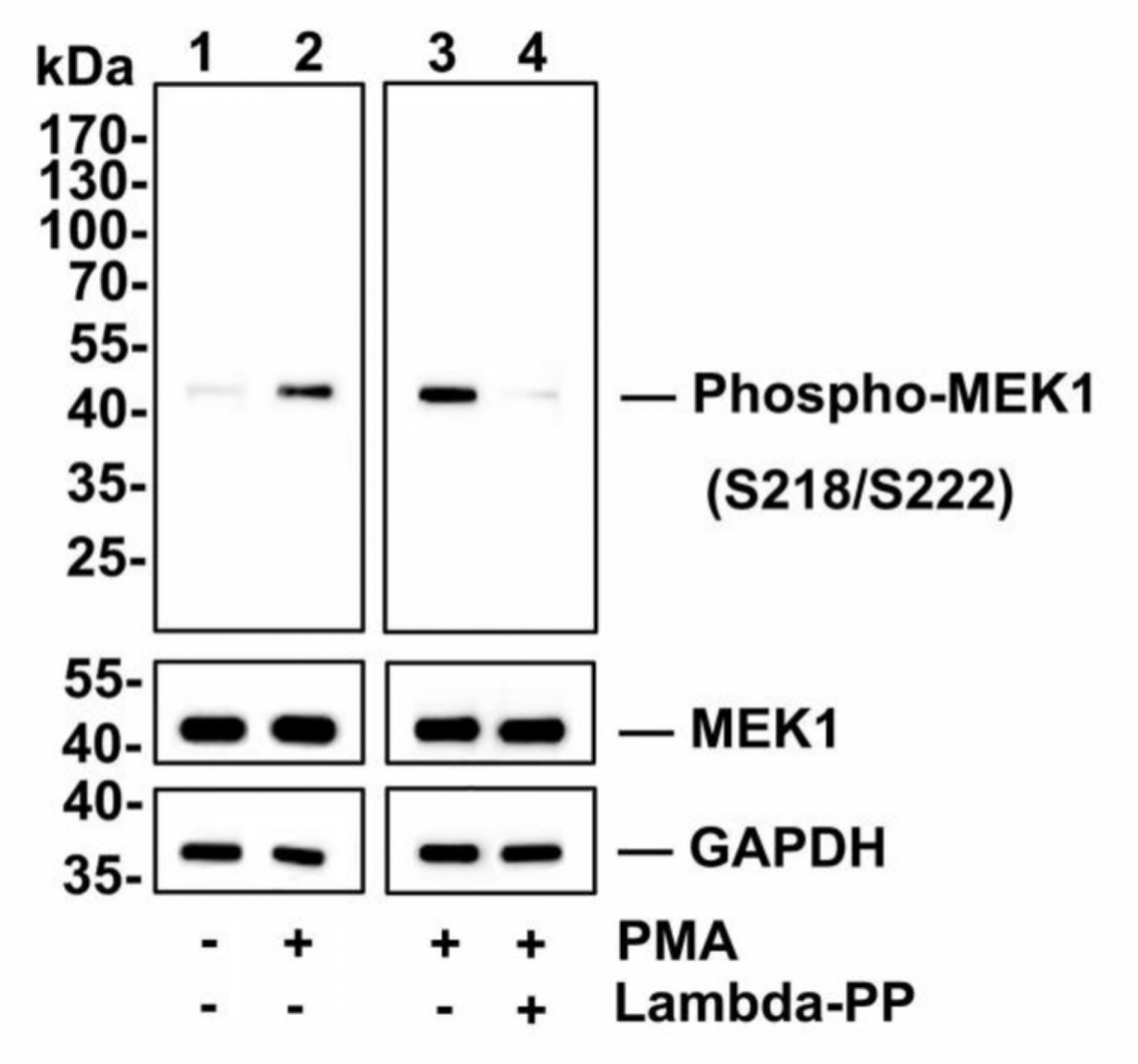
Multiple Antibody Strategy
Validate using two or more independent antibodies that can recognize different antigenic determinants of the target protein.
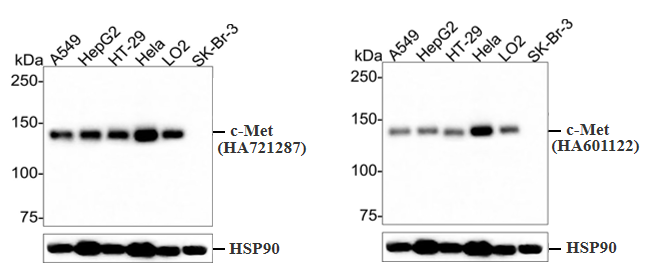
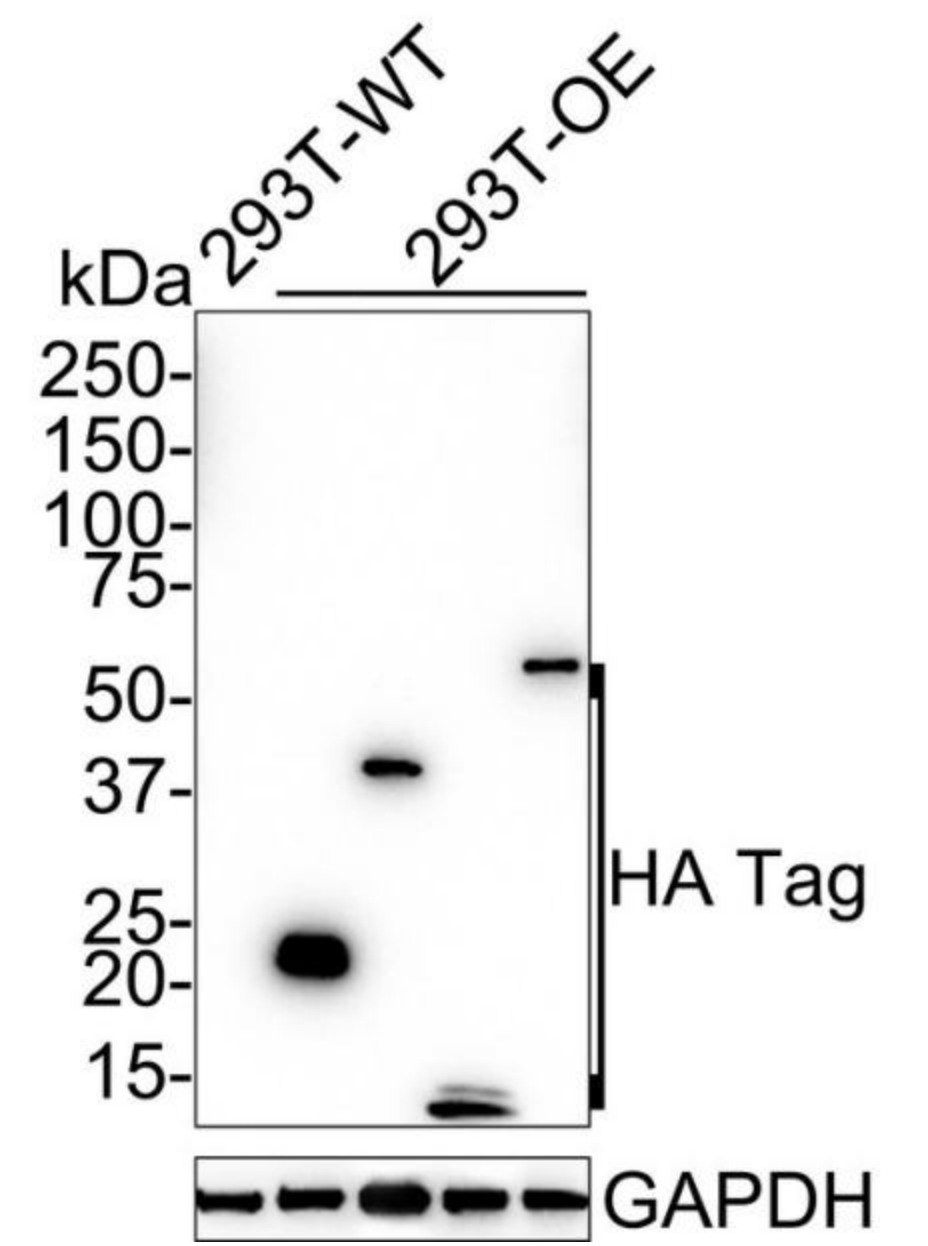
Heterologous Expression Strategy
Overexpress the natural (or mutant) target protein in a cell line to validate the positive signal of the antibody.
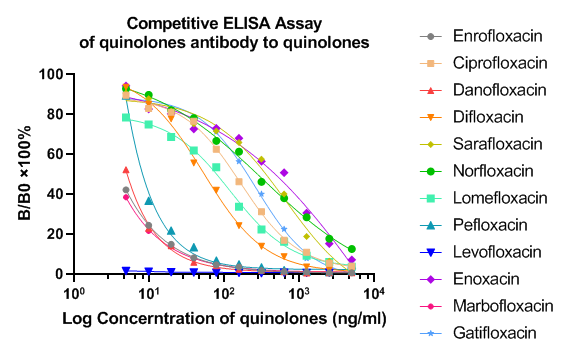
Supplementary Validation Strategy
Use complementary assays to validate antibody specificity, including competitive ELISA, peptide blocking, or protein arrays.
Recombinant Production Strategy
Produce antibodies using a recombinant technology platform, which speeds up antibody production and ensures batch - to - batch stability of antibodies.
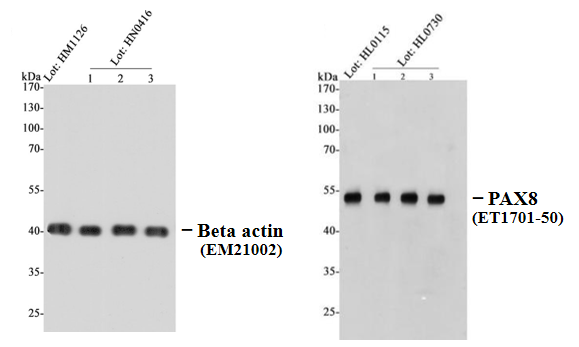
Batch - to - Batch Consistency
HUABIO evaluates each new batch. After each new batch is successfully produced, we compare the latest batch with the previous ones. We use the same experimental conditions to review the consistency of activity and specificity.
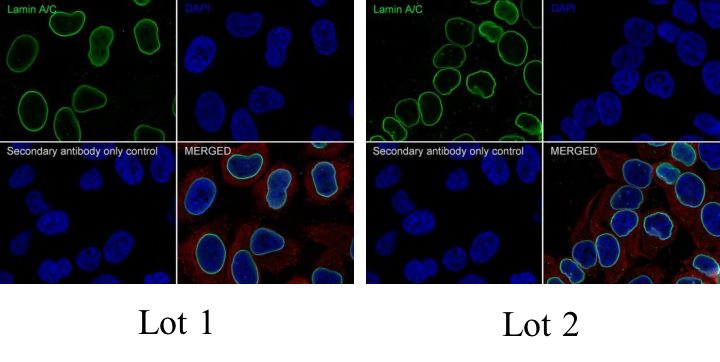
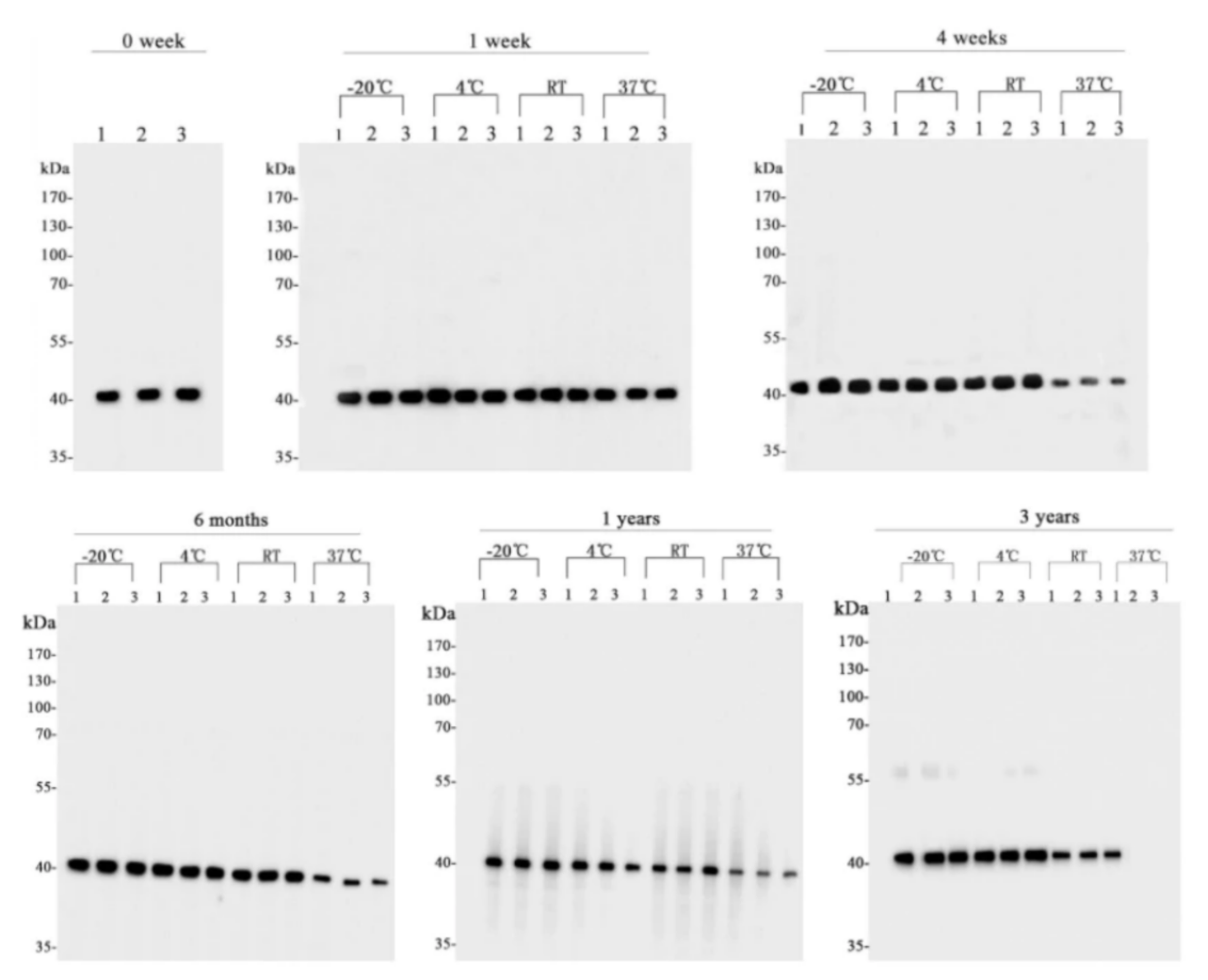
Antibody Stability
We have examined the stability of antibodies at different storage temperatures and for different lengths of time to ensure the stability and quality of antibodies during transportation and storage. It has been proven that the activity of some of HUABIO's antibodies remains intact at room temperature for up to three months.
Product shown: Beta - Actin Monoclonal Antibody, [ EM21002 ] 1:1,000 <br /> Sample used: PC - 12 whole cell lysate
